The Preterm Anthropometric Growth Apparatus (PAGA) addresses a critical need in neonatal care: the accurate and consistent measurement of anthropometric data. In collaboration with Innovus, the technology transfer office of Stellenbosch University, we developed an all-in-one, and cost-effective tool designed to optimise the monitoring of preterm infant growth and support improved developmental outcomes.
PAGA
Overview

Challenge
To perform anthropometric measurements in preterm infants, babies are typically removed from their incubators, which exposes them to increased risks of temperature loss, infection, and physiological stress - all of which can negatively impact their clinical stability and growth. These risks are especially concerning during the early, most fragile days of life.
For infants receiving nasal continuous positive airway pressure (nCPAP), removal from the incubator is often not possible, as they must remain connected to life-sustaining respiratory support. As a result, these infants are frequently excluded from routine growth monitoring, particularly during the critical window when early nutritional and medical interventions could significantly influence outcomes. This means that, paradoxically, the most vulnerable infants (those at highest risk of growth faltering) miss out on essential measurements that could inform their care.
Furthermore, traditional measurement methods typically only assess a single parameter at a time (e.g., head circumference or length), which requires multiple tools and repeated handling. This further increases stress for the infant, workload for NICU staff and can lead to inconsistent and incorrect measurements, therefore limiting the ability to obtain reliable, comprehensive growth data.
Objective
The key objective was to develop a device that:
- Simultaneously measures multiple anthropometric parameters with clinical accuracy.
- Is minimally invasive for the infant and user-friendly for the medical personnel.
- Can connect with a mobile application to analyse and interpret measurements on growth charts.
- Ensures compatibility with existing NICU equipment, including open and closed incubators.
Solution
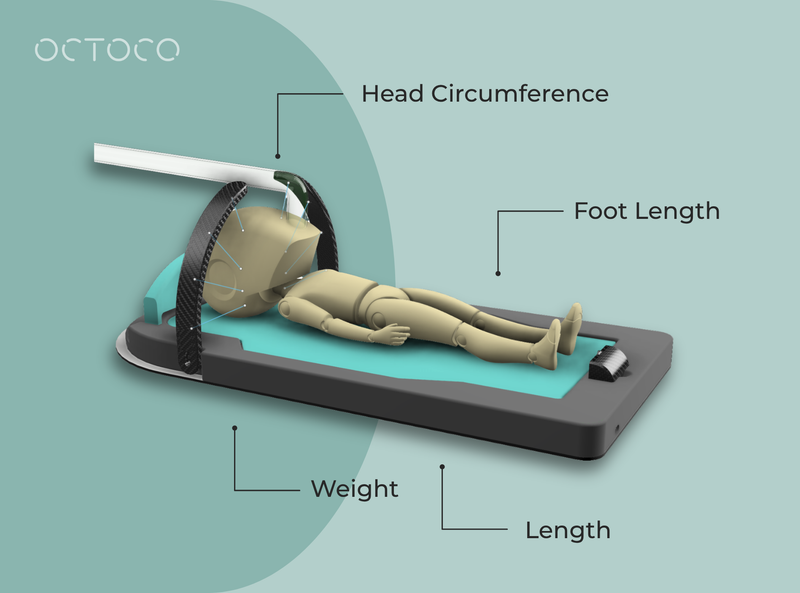
The PAGA was designed as a multi-parameter anthropometric growth apparatus that integrates various measurement capabilities into a single device. This all-in-one solution enables healthcare providers to measure an infant’s weight, length, head circumference, and foot length without disconnecting them from life-supporting equipment. The device slides under the infant in their incubator and takes all the measurements in a non-invasive way. The solution also features Bluetooth connectivity, allowing real-time data visualisation and cloud storage for seamless digital growth tracking. An integrated algorithm calculates gestational age, further aiding in growth monitoring and clinical decision-making.
Implementation
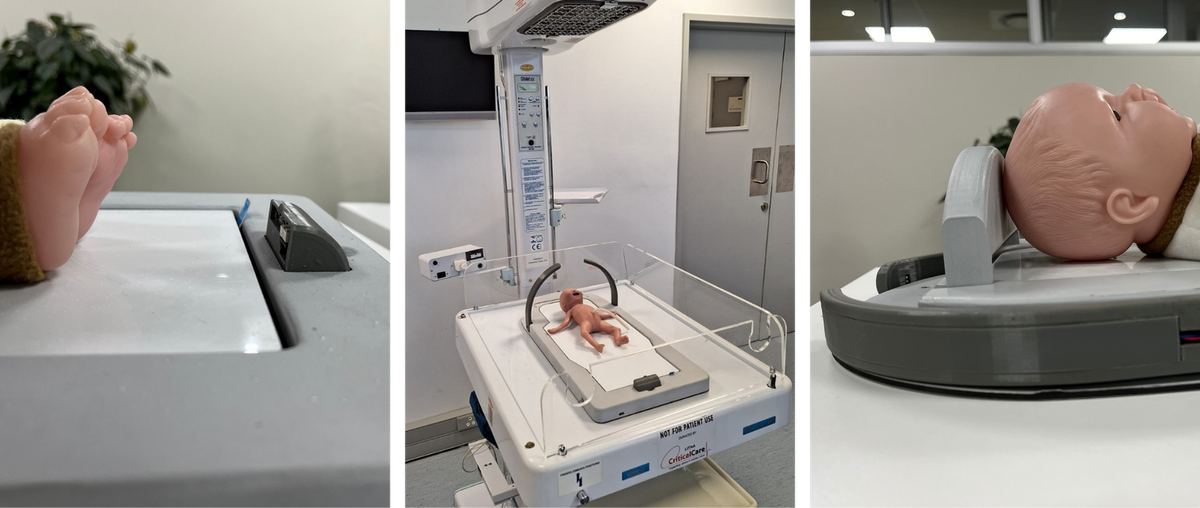
A beta prototype was developed and 3D-printed, featuring a modular design with five key components: the shell, base plate and infant bed, halo, length-sensing pop-up, and headrest. The device is equipped with time-of-flight sensors and other measurement methodologies to ensure precision.
In addition to the device, Octoco also developed a mobile application to provide real-time analysis, standardised digital growth charts, and comprehensive growth interpretations. We implemented embedded software to control device operation, process sensor inputs, and calculate accurate measurements while ensuring system reliability.
Result
The PAGA prototype successfully demonstrates the ability to measure multiple anthropometric parameters without removing infants from incubators or disconnecting support equipment. The system's all-in-one approach significantly reduces measurement time and potential stress to infants while increasing data accuracy and consistency.
The PAGA solution is now positioned for clinical validation and commercialisation, with potential markets including neonatal ICUs in both private and state hospitals in South Africa. They are currently seeking commercial partners, philanthropic funders, and social impact investors to help bring this life-saving technology to market.